SICOMED, your market access sherpa for Switzerland
SICOMED supports pharmaceutical companies in navigating the complexity of Swiss market access, from the launch of innovative therapies to successful life-cycle management.
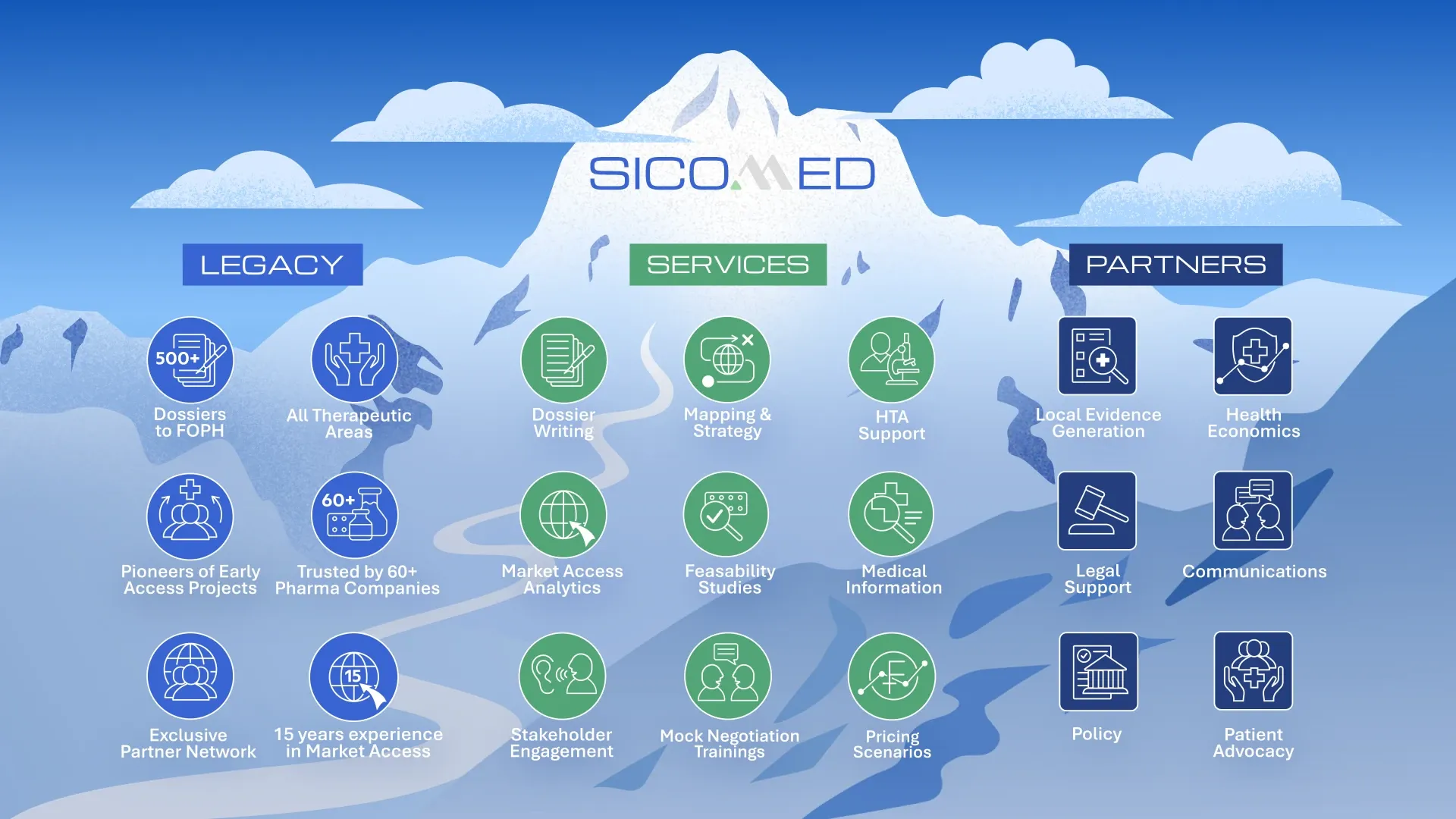
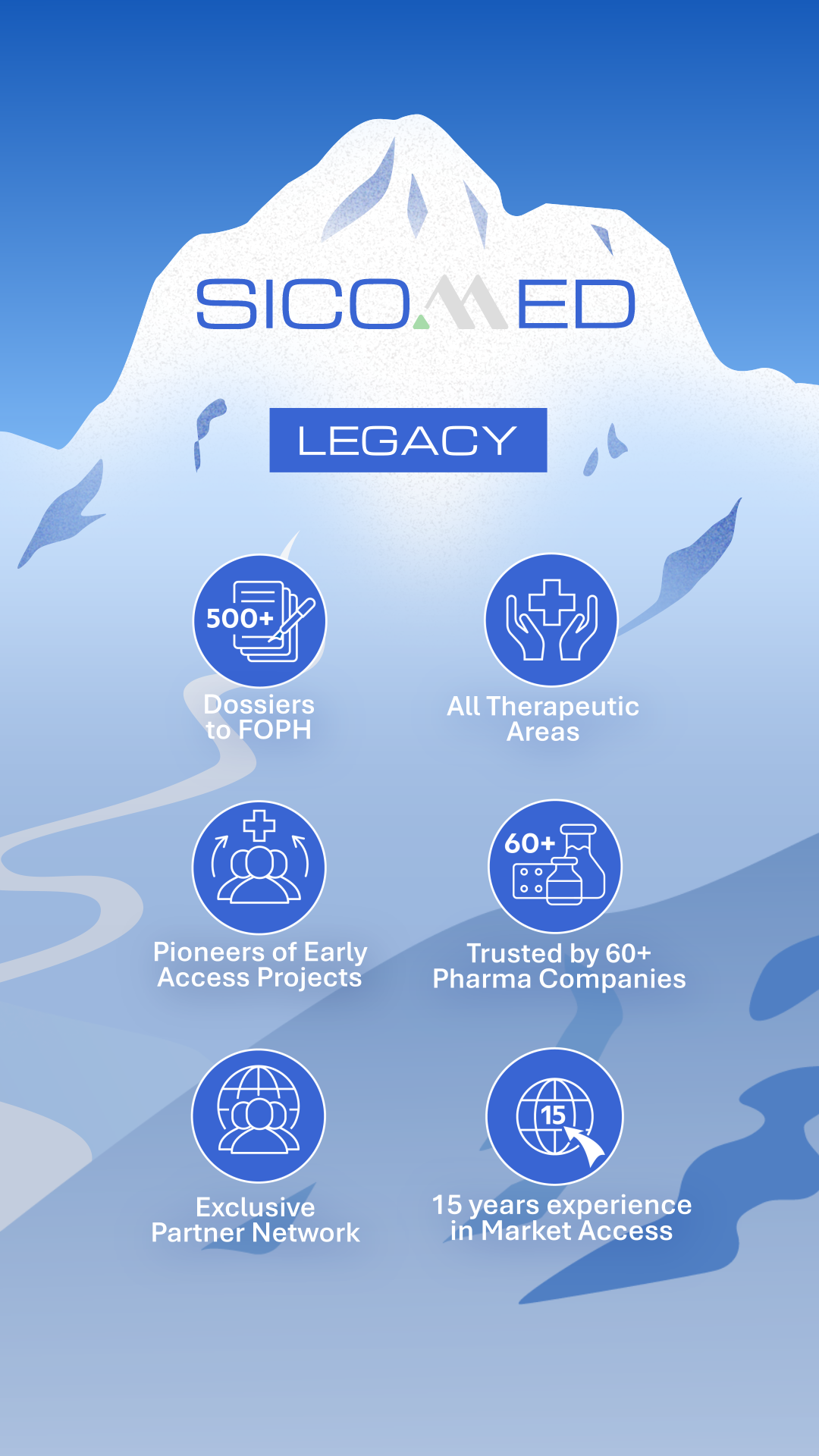
With over 500 dossiers submitted to the Federal Office of Public Health (FOPH) and the trust of more than 60 pharmaceutical companies across all therapeutic areas, we ensure transparency and efficiency in pricing and reimbursement (P&R) processes.
“Navigating the Swiss healthcare system is challenging. Rising costs, complex regulations, and lengthy reimbursement processes require in-depth expertise and precise guidance.“
At a glance – who we are and what we offer
Services
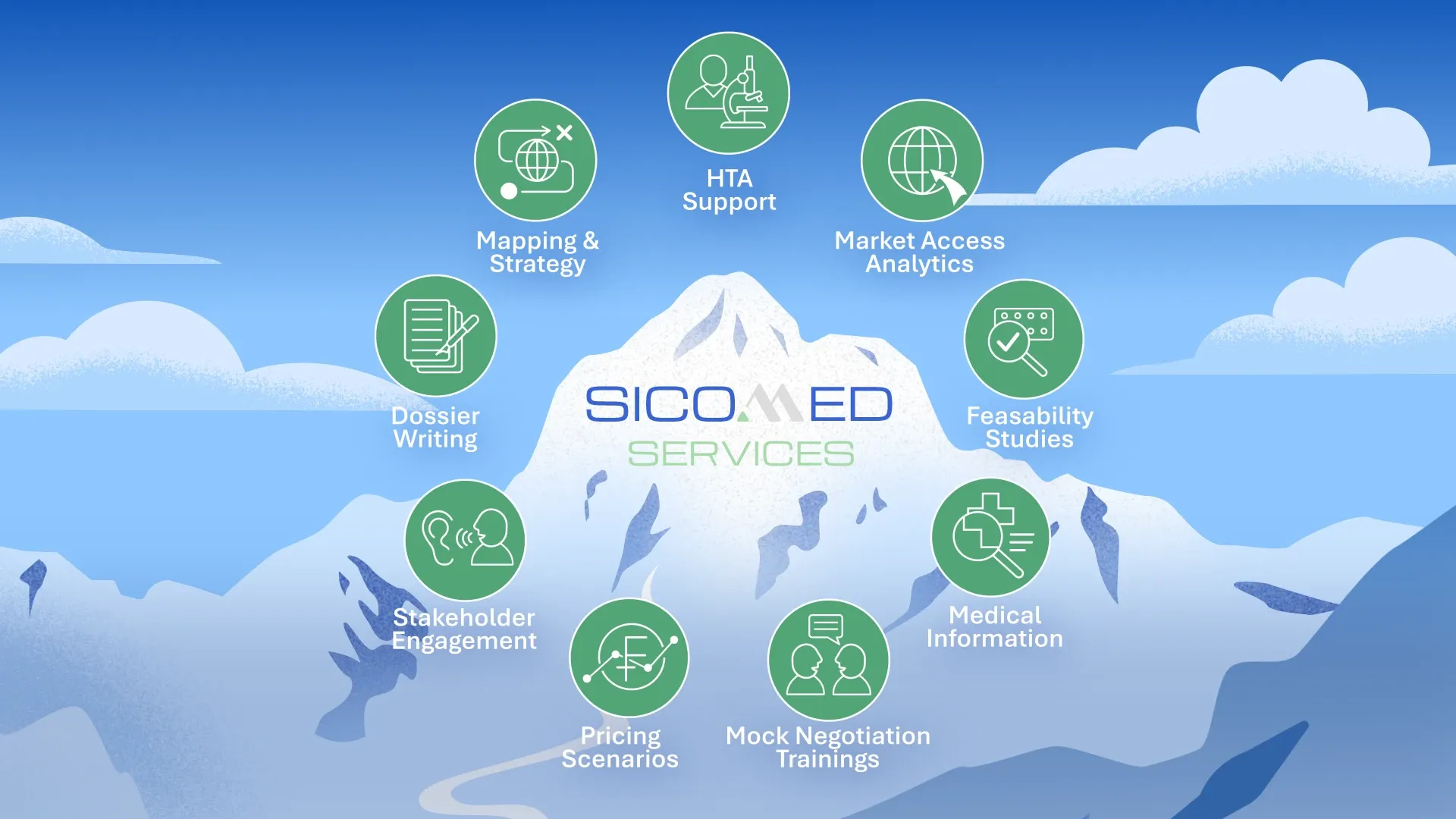
Our expertise covers the full spectrum of Swiss market access and beyond:
- Market access strategy & implementation
- Pricing & reimbursement submissions (scenario modeling, dossier preparation, 3-year price reviews, price increase applications, etc.)
- Pre-launch and launch activities, including Art. 71 and early access programs
- Health Technology Assessment (HTA) support
- Negotiation and policy support (mock negotiation trainings)
- Stakeholder engagement, patient advocacy & communication
- Evidence generation and health economics
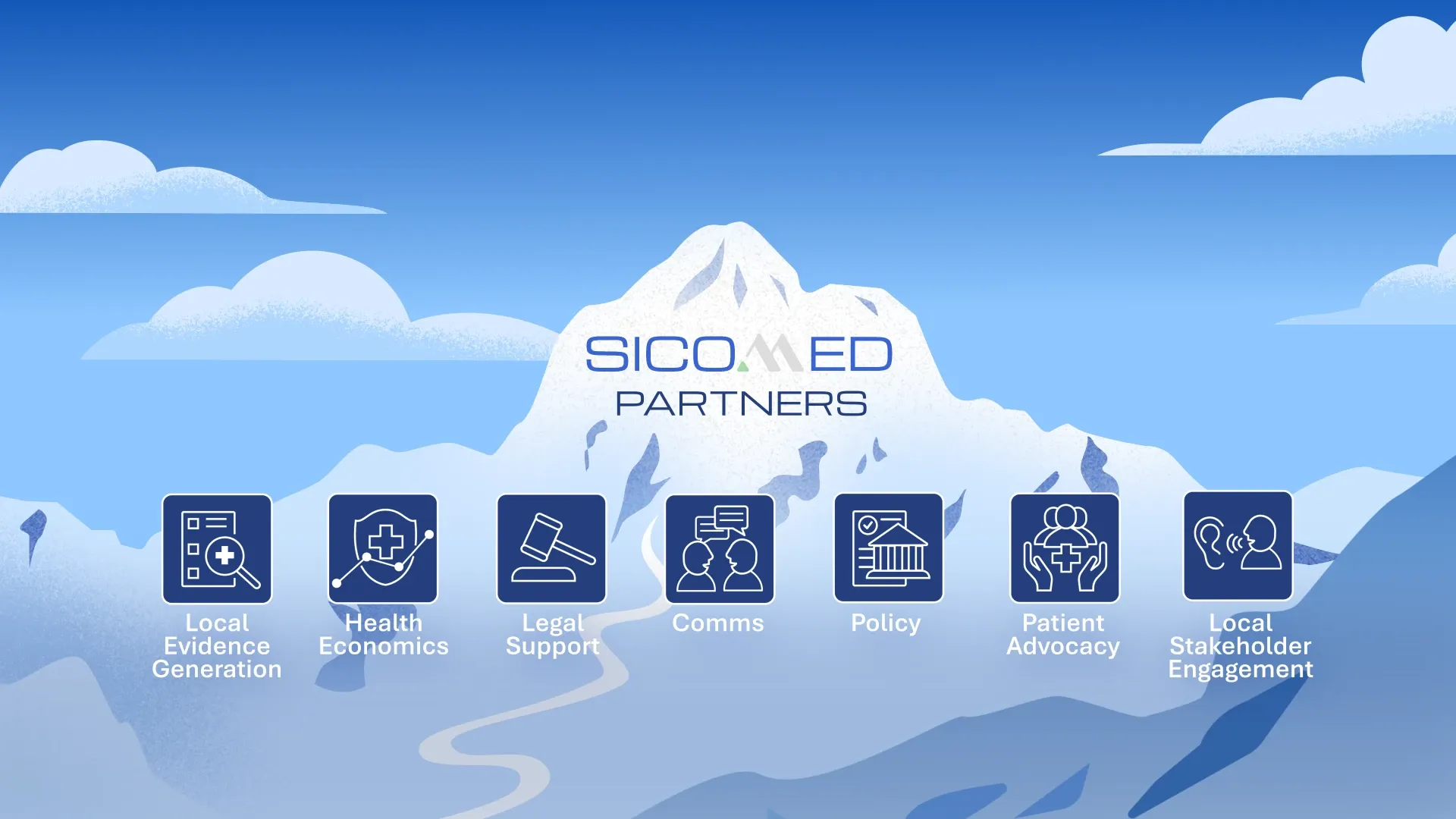
Partners
We work with a trusted network of experts in the fields of law, policy, and communication to deliver tailored solutions that go beyond the core business of market access.
Within the Accessus Health network, we combine Swiss precision with European reach, providing comprehensive market access expertise from planning through to implementation.
About Petra Erni
Petra is an experienced market access specialist with over 15 years of experience advising pharmaceutical companies and healthcare organizations across all therapeutic areas in Switzerland. She combines regulatory expertise, strategic foresight, and a broad network within the Swiss market access landscape.
Her work focuses on pricing and reimbursement processes at the Federal Office of Public Health (FOPH) — from strategic planning and dossier preparation to support with price negotiations, evidence generation, early-access projects, and price reviews. She is therefore one of the few experts who master Swiss reimbursement processes from A to Z.
In addition to her consulting work, Petra is also a lecturer in leading market access courses in Switzerland, including SHQA and MEGRA, where she shares her expertise with the next generation of market access professionals.
Before founding SICOMED, Petra held leadership positions in the pharmaceutical industry, including as Business Unit Head at GSK (GlaxoSmithKline). On the payer side, she worked in the Pharmaceuticals Section of the FOPH, where she analyzed reimbursement applications and negotiated drug prices.
References
“Petra combines outstanding expertise in the field of market access with an authentic and clear personality. She advised us with an excellent product potential and scenario analysis for our dossier submission and guided us competently through the pricing and reimbursement process. We particularly appreciate her reliability, open and honest communication, as well as her ability to present complex issues in a simple way and never make things unnecessarily complicated.”
Head of Market Access
Top-10 Pharmaceutical Company
“We have come to know SICOMED as a highly trustworthy and competent partner. Petra knows the challenges of the Swiss system inside out and handles them with impressive ease. With her network of relevant stakeholders and experts, she brings in the right partners as needed — from stakeholder engagement to legal issues, policy communication, and evidence generation. Petra supported us in the framework of an Early Dialogue/FOPH Early Access process as well as in the Article 71 process, and she prepared us specifically for a demanding negotiation. For us, she was always a personable and extremely effective advisor.”
CEO
Small Biotech Company
“We have been working successfully with Petra for many years and greatly value her extensive expertise in market access. As an external market access specialist, she provides comprehensive advice on all questions related to the reimbursement of our medicines in Switzerland. She competently supports us in the preparation of applications as well as in the life cycle management of our established products.
Petra is reliable, pragmatic, and highly experienced. She responds quickly and solution-oriented, even to short-notice requests, and delivers results we can depend on. Thanks to her solid knowledge of regulations, she helps us bring the critical points to the table clearly and efficiently. Her down-to-earth and modest style makes collaboration always pleasant and straightforward.”
GM AT/CH
of a mid-sized pharmaceutical company